Regulatory Writing
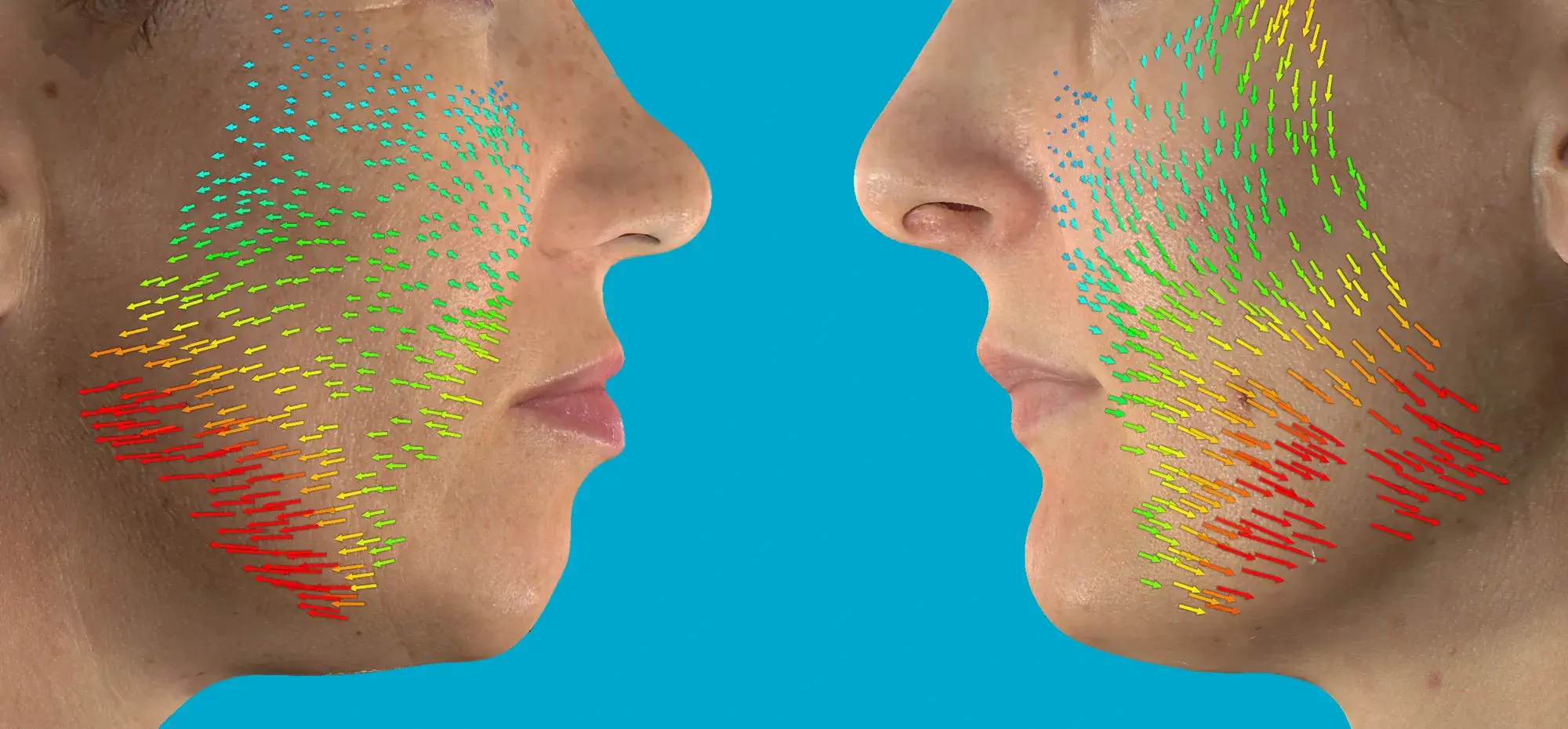
Provided with courtesy of Dr. Lukasz Preibisz
Dealing with clinical documents required by ethics committees, health authorities or market access agencies
- Study synopsis, protocols/CIP and amendments
- Clinical investigation/study reports (CIR/CSR)
- Case report forms (CRF)
- Ethics committees- and health authorities application forms
- Investigator’s brochures (IB)
- Patient brochures
- Patient information sheets
- Patient informed consent forms (ICF)
- Manuals of operations
- Clinical evaluation reports (CER) for medical devices
- Answering to deficiency letters
- Product labelling: summary of product characteristics (SmPC) and instructions for use (IFU)
- Clinical modules of marketing authorisation applications (clinical summary and overview, summary of safety and efficacy) for European notify bodies and FDA
Scope of clinical studies:
- Phases I-IV studies (pharmaceuticals drugs)
- Pilot/pivotal/PMCF studies (medical devices)
- Non-interventional studies/programs (NIS/NIP)
- Registries
- Investigator-initiated trial (IIT)